Instrumental Analysis of Chemical Elements
Quality Control - The Terms Precision and Accuracy
In addition to the similar need for highest representative quality of the sample to be analysed or to be used as a bioindicator, most general rules and prerequisites of quality control in chemical analysis have to be taken into account in biomonitoring activities. In the last 20 years a strict differentiation between the terms "precision" (reproducibility) and "accuracy" (the "true" value) has been established in chemical analytical research. The practical application of this differentiation makes it possible to determine the "true" or real content of a substance "X" in a sample "Y". The purpose of determining the precision of the data by repeatedly measuring the analytical signal is to track down and eliminate errors which might be generated, for example, by insufficient long-term stability of the measuring device (device-specific misadjustment). If the analytical procedures are not too complex, the precision will be 1 to 5 %, and for most analytical problems this can be considered sufficiently exact. However, the mere fact that a signal is readily reproducible does not permit any statement about its accuracy. Even highly precise data can diverge greatly from the "true" (e.g. element) content of a sample.
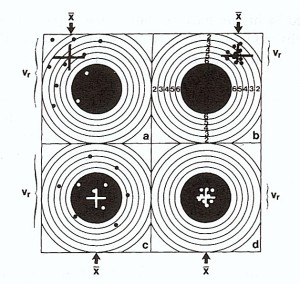
Figure: Illustration of the terms "precision" (reproducibility) and "accuracy" (the "true" value) in analytical chemistry (after Toelg, 1976 from Markert, 1996): a. Poor precision and poor accuracy, b. good precision and poor accuracy, c. poor precision and good accuracy, d. good precision and good accuracy, = arithmetic mean, vr = coefficient of variation.
Correct analytical results can only be obtained if the entire analytical process is subjected to targeted quality control, where every result is checked for its precision and accuracy. Basically, two methods are now used to check the accuracy of analytical results: a) use of standard reference materials (commercially available samples with a certified content of the compound to be measured and a matrix similar to the original samples to be measured in the laboratory) and b) use of independent analytical procedures.